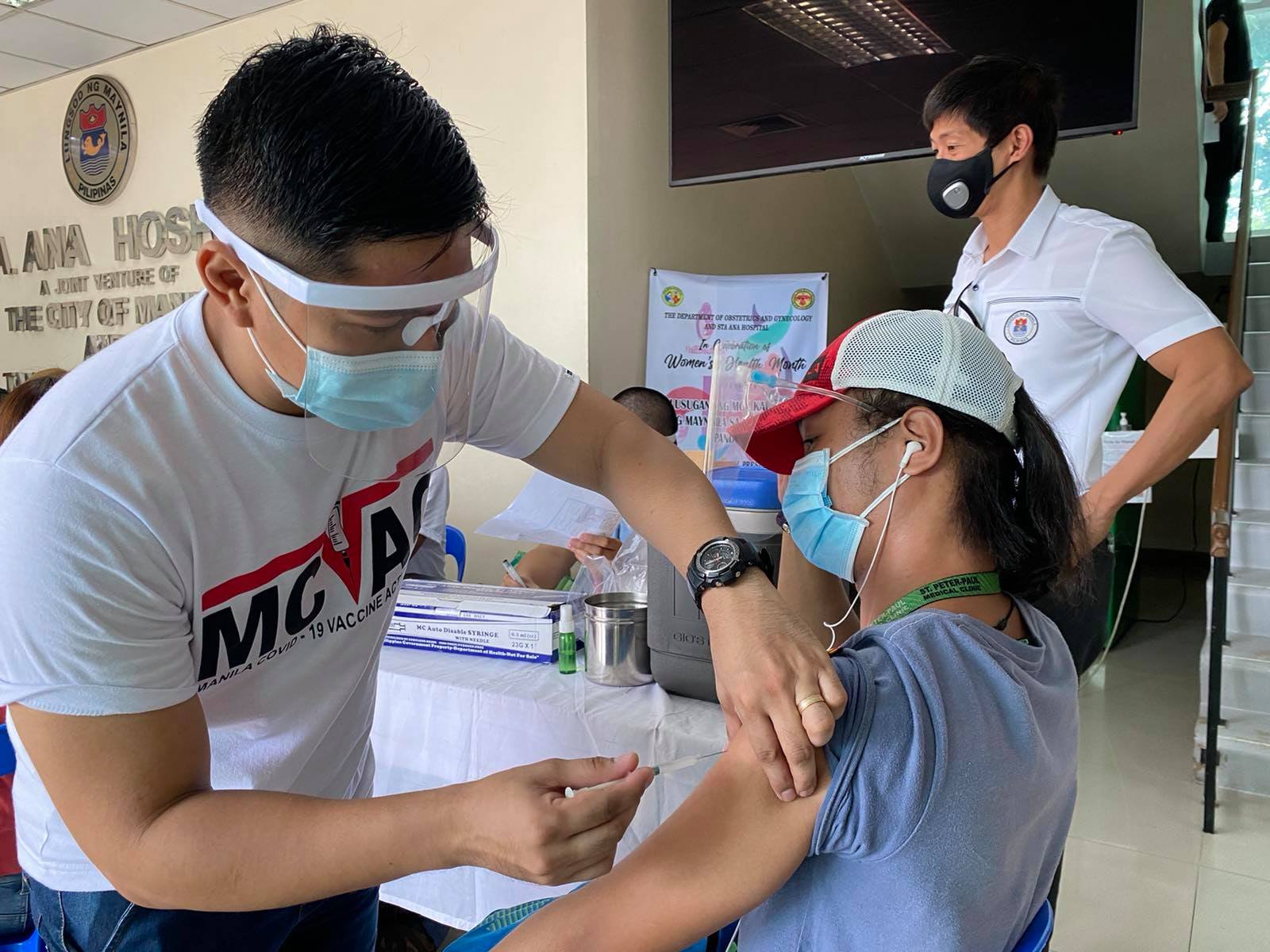
Regulators have approved the administration of Covid-19 booster shots for the general adult population, Food and Drug Administration (FDA) chief Eric Domingo said on Wednesday.
In a text message to reporters, Domingo said benefits of booster shots outweighed the known and potential risks.
“Approved on Monday to include all adults 18 years old and above, based on assessment of benefit and risks,” said Domingo.
The following are the vaccine brands for heterologous booster shots:
- Sinovac (primary series) — AstraZeneca, Pfizer, Moderna (booster)
- AstraZeneca (primary series) — Pfizer, Moderna (booster)
- Pfizer (primary series) — AstraZeneca, Moderna (booster)
- Moderna (primary series) — AstraZeneca, Pfizer (booster)
- Gamaleya (primary series) — Pfizer, AstraZeneca, Moderna (booster)
- Janssen (primary series) — AstraZeneca, Pfizer, Moderna (booster)
Domingo said the Department of Health would issue the guidelines for the administration of booster shots for the general adult population.
Health Undersecretary Myrna Cabotaje said these could be completed “within the next couple of days.”
The Philippines started administering booster shots on Nov. 17.
It has deployed 313,836 booster doses as of Nov. 30. John Ezekiel J. Hirro